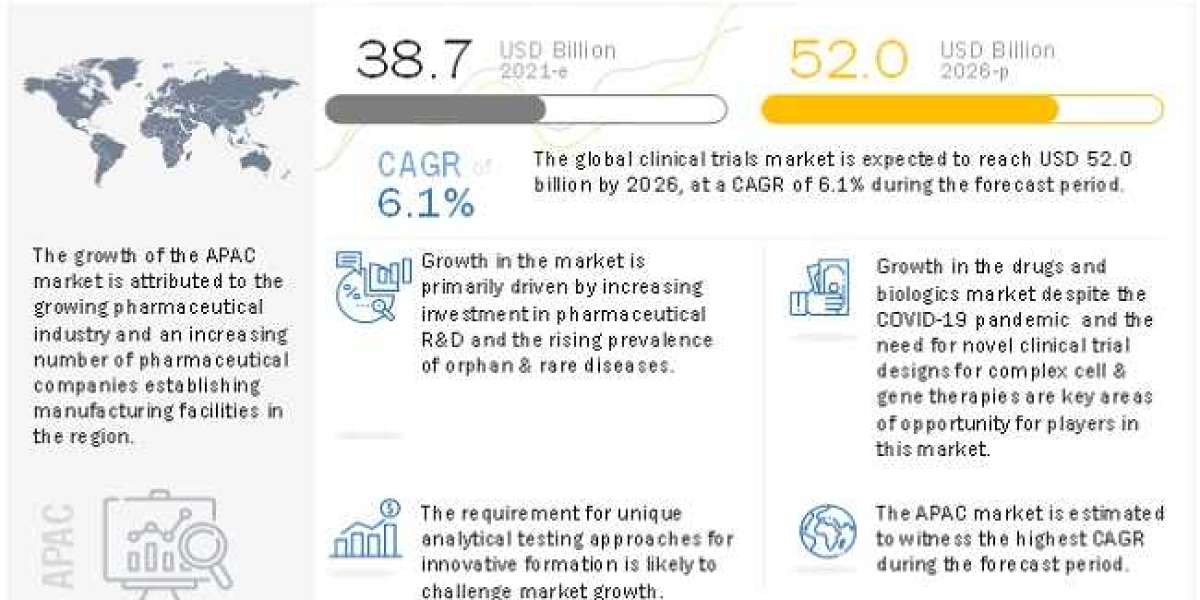
The Global Clinical Trials Market is projected to reach USD 52.0 billion by 2026 from USD 38.7 billion in 2021, at a CAGR of 6.1% during the forecast period. The growing demand for biosimilars and biologics, rising adoption for specialized testing services, and increasing preference for outsourcing clinical trials from emerging Asian markets offer significant growth opportunities for players operating in the market.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=405
Browse in-depth TOC on "Clinical Trials Market”
324 – Tables
59 – Figures
304 – Pages
Key Market Players
The prominent players operating in the clinical trials market are IQVIA (US), LabCorp (US), PPD (US), PRA Health Sciences (US), Syneos Health (US), Charles River Laboratories (US), WuXi AppTec (China), Paraxel International (US), and ICON Plc (US).
Enquiry Before Buying this Report:https://www.marketsandmarkets.com/Enquiry_Before_BuyingNew.asp?id=405
Market Trends: Adoption of artificial intelligence-based tools for drug discovery
Artificial intelligence (AI) and machine learning (ML) make the drug discovery process more efficient and substantially improve success rates at the early stages of drug development. AI algorithms ingest and analyze a vast amount of information and can identify potential drug candidates in shorter periods of time. Deep learning systems can also be used for generating molecules with properties that are likely to be effective against specific diseases without adverse side effects. Such advantages have encouraged the adoption of AI for drug discovery by various pharmaceutical and biotechnology companies. For instance, Benevolent Ltd. (UK) focuses on applying deep learning and natural language processing to understand and analyze large volumes of information for drug discovery.
By phase, Phase IV is expected to grow at a fast pace during the forecast period.
Based on phase, the clinical trials market is segmented into Phase I, Phase II, Phase III, amp; Phase IV. Phase III dominated the clinical trials market in 2020, whereas Phase IV registered highest growth rate during the forecast period. Variety of formulations, therapy duration, dosages, and other interactions are studied in this phase as a part of post-marketing surveillance. Increase in number of service providers offering post-marketing surveillance has contributed to the highest growth rate registered by this segment.
By service type, the laboratory services segment accounted for the largest share of the clinical trials market in 2020.
Based on service type, the clinical trial services market is segmented into protocol designing, site identification, patient recruitment, laboratory services, bioanalytical testing, analytical testing, clinical trial supply amp; logistic services, decentralized clinical services, clinical trial data management services, medical device testing services, and other services. Laboratory services are used to provide support to all stages of the drug development process, including clinical development. The quality and efficacy of the products are determined through various qualitative and quantitative processes.
By therapy area, oncology segment dominated the clinical trials market in 2020
Based on the therapy area, the clinical trials market is segmented into oncology, infectious diseases, cardiology, neurology, women health, genetic diseases, immunology, and other therapy areas. Oncology segment dominated the clinical trials market in 2020. The list of drugs in development for oncology has increased over the years due to the rising number of clinical trials and growing RD expenditure by pharmaceutical companies on oncology-based drugs.
By application, the vaccine segment is set to grow at the fastest pace through the forecast period.
Based on application, the global clinical trial market is segmented into small applications, vaccines, cell amp; gene therapy, and others. Companies are enforcing their expertise on COVID-19 vaccine trials to drive the successful commercialization of COVID-19 vaccines. Approximately 194 COVID-19 vaccine candidates were in pre-clinical development, whereas 138 were in the clinical development phase as of January 2022. This has contributed to the segmentrsquo;s fastest growth.
Recent Developments:
- In September 2021, Syneos Health entered into a strategic collaboration with Ride Health to offer non-emergency medical transportation (NEMT) for clinical trial participants.
- In April 2021, IQVIA acquired Q2 Solutions, a clinical laboratory services organization, from Quest Diagnostics.
- In November 2020, WuXi Apptec expanded its Cell amp; Gene Therapy Platforms with capabilities to provide high-quality and cost-effective supplies of Ramp;D and GMP Plasmids
- In April 2020, IQVIA launched COVID-19 trial matching service to accelerate treatment and vaccine development against the COVID-19 pandemic in U.S. The company launched comprehensive online screener and trial matching tool for all COVID-19 trials in the US.
- In May 2020, IQVIA announced the Japan and Asia Pacific expansion of IQVIA Biotech to deliver integrated clinical solutions and support biotech and emerging biopharma companies.
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=405
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their pain points around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 Micro Quadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, "Knowledge Store" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Research Insight: https://www.marketsandmarkets.com/ResearchInsight/clinical-trials-market.asp
Visit Our Website: https://www.marketsandmarkets.com/
Content Source: https://www.marketsandmarkets.com/PressReleases/clinical-trials.asp
Related Report: