Do you know sodium which is the most reactive metal? No? It is potassium! Potassium is like sodium, i.e., very reactive, soft, and vigorous. Due to its reactivity, it is kept in kerosene. Similar to sodium, potassium also reacts with oxygen and forms potassium oxide. But can you answer, is potassium oxide ionic or covalent? Is this compound acidic or basic? What are the applications of potassium oxide? This section will let you solve all such queries about potassium oxide.
What Is Potassium Oxide?
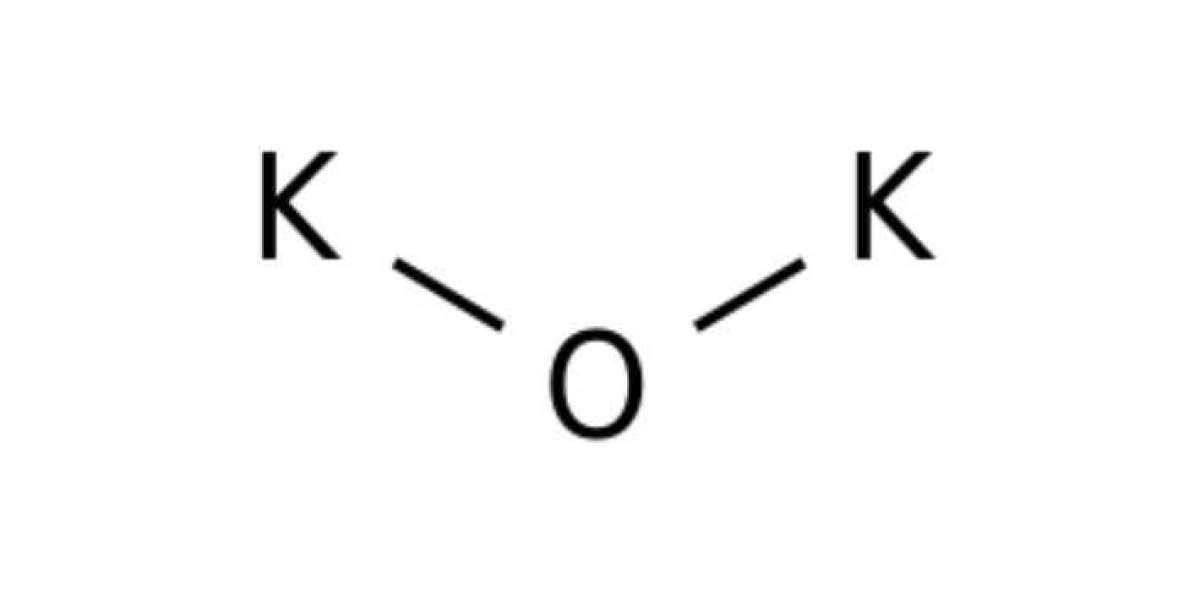
It is a compound formed by the bonding between oxygen and potassium. It is also known as dipotassium oxide or potassium monoxide. It is an inorganic compound. Like sodium oxide, it is also a highly reactive and rarely encountered compound. It is easy to guess whether it is ionic or covalent. Firstly, potassium is a metal, and metals are electropositive, while oxygen is one of the highly electronegative elements. So, polarisation occurs, and the compound formed is ionic. Secondly, it is explained by the potassium oxide Lewis structure, which is explained further.
Structure and Formula of Potassium Oxide
The molecular formula of it is K₂O. In general terms, it is called alkali metal oxide. It is the simplest compound of potassium that is highly reactive. This compound is an ionic compound. It is because potassium has only one electron in its outermost shell, and oxygen is short of two electrons to gain a fulfilled octet. Hence, two potassium atoms will donate their electrons to the oxygen atom and form ionic bonds. As a result, the compound form is also ionic.
Physical Properties of Potassium Oxide
Potassium is a member of the sodium family, i.e., group I in the periodic table. It tends to complete its octet by releasing electrons. Therefore, it is highly reactive in its free form. When it is treated with oxygen, it readily makes a bond with the O-atom and forms potassium oxide.
Conclusion
After going through the above article, you are now well informed about potassium oxide. It is an inorganic acid with a highly reactive characteristic property. The formula of potassium oxide is K₂O. It has a basic or alkaline nature. It gives neutralization reactions when treated with strong acids. Due to its different properties, it is used majorly as a fertiliser in agriculture. It is useful in the glass, ceramic, and optic industries. It becomes toxic when ingested and inhaled. Due to its toxic nature, it is necessary to take precautions while working with it.