Global Lentiviral Vector Market research report contains the most recent market information with which companies can get in depth analysis of Healthcare industry and future trends. Wide-ranging evaluation of the market growth predictions and restrictions has also been studied in this report. This market report offers wide-ranging analysis of the market structure for Healthcare industry along with estimations of the various segments and sub-segments of the market. It offers key measurements, status of the manufacturers and proves to be a significant source of direction for the businesses and organizations. With an international Global Lentiviral Vector Market report business can construct a strong organization and make better decisions that take business towards the great level of success.
Request Sample of Lentiviral Vector Market Report @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-lentiviral-vector-market
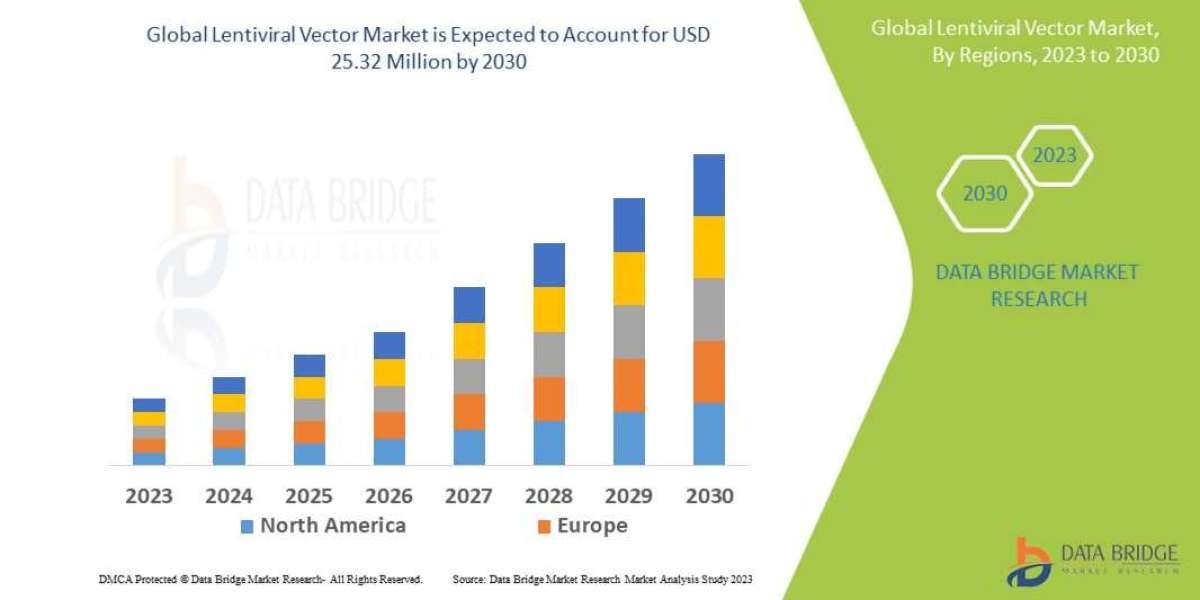
Lentiviral Vector Market Dynamics
Drivers
- Increasing research and development activities
Various manufacturers are conducting RD for novel lentiviral gene therapies, which is expected to drive growth in the global lentiviral vectors market. For instance, AVROBIO, Inc., a clinical-stage gene therapy company, presented pre-clinical data for AVR-RD-03 for Pompe disease on May 14, 2020. The research demonstrated the efficacy of lentiviral gene therapy for Pompe disease manifestations in the muscle and central nervous system (CNS).
- Increasing agreements and collaborations
The lentiviral vectors market is expected to grow due to key players focusing on various inorganic growth strategies such as agreements and collaborations. For instance, bluebird bio, Inc. signed a licence agreement with Novartis Pharma AG in May 2017. Novartis will non-exclusively licence certain bluebird patent rights related to lentiviral vector technology in order to develop and commercialise oncology therapies.
Opportunities
- Lentiviral vector demand for t cell engineering for cancer treatment surging
Lentiviral vectors have already been helpful in forming gene-modified cell-based therapies, particularly T cell therapies. Such vectors are also used to deliver Chimeric Antigen Receptors (CARs) or cloned T-cell receptors to mature T cells to induce anti-cancer immunity. The FDA approved the first genetically engineered cellular therapy using lentiviral vectors after CAR T-cell therapies generated with lentiviral vectors demonstrated significant clinical efficacy in patients with B-cell malignancies. Lentiviral vectors have changed and evolved alongside CAR T-cell therapy, and they are now demonstrating their full potential as an alternative therapy and a powerful, supplemental tool in the treatment of a broader range of cancers.
Restraints/Challenge
- Lack of scalability and downstream purification
The lack of scalability and downstream purification limitations of lentiviral vectors may impede the future growth of a lentiviral vector market. Strategic initiatives by major market players and increased funding for genomics provide opportunities for the global lentiviral vector market to grow. The stringent regulatory policies and side effects associated with lentiviral vector products pose a challenge to market growth in the future.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The lentiviral vector market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for lentiviral vector market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the lentiviral vector market.
Recent Developments
- In 2020, Waisman Biomanufacturing entered into a strategic partnership with the California-based biotech company GigaGen to develop a new drug to treat and prevent COVID-19. This strategic partnership formed by the company will increase the use of development and testing of new types of drugs by Waisman Biomanufacturing, resulting in increased market awareness of its services.
- In 2021, Oxford Biomedica signs a new three-year Development Supply Agreement with Boehringer Ingelheim to manufacture and supply viral vectors. This agreement is expected to boost the company's sales growth in the coming years.
Profound Questions Answered in this Report:
- What was the size of the emerging Lentiviral Vector market opportunities, market risk and market overview by value in 2023?
- What factors are affecting the strength of competition in the emerging Lentiviral Vector market?
- How large is the emerging Lentiviral Vector market in relation to its regional counterparts?
- What are the developments in the global market and forecast of market size up to 2030?
- What will be the share of the emerging Lentiviral Vector market in 2030?
- What is the Lentiviral Vector Market size in different countries around the world?
- Are the markets growing or decreasing and company profile including analysis of product portfolio, revenue, SWOT analysis and latest developments of the company?
- How are the market opportunities and threats faced by the vendors in the Lentiviral Vector industry?
- How are different product groups developing?
- Who are the distributors, traders and dealers of Lentiviral Vector market?
- How are the markets forecast to develop in the future?
A list of all the renowned Lentiviral Vector Key Players in the market:
- Cobra Biologics Limited (U.K.)
- Sirion-Biotech GmbH (Germany)
- Merck KGaA (Germany)
- FinVector Oy (Finland)
- Oxford Biomedica (U.K.)
- OriGene Technologies, Inc. (U.S.)
- Sino Biological Inc. (China)
- Cell Biolabs, Inc. (U.S.)
- Batavia Biosciences B.V. (Netherlands)
- Lonza (Switzerland)
- GENEMEDI (China)
- Takara Bio Inc. (Japan)
- Thermo Fisher Scientific Inc. (U.S.)
- Waisman Biomanufacturing (U.S.)
- Cytiva (U.S.)
Request for PDF Brochure of This Report@ https://www.databridgemarketresearch.com/reports/global-lentiviral-vector-market
Browse Related Reports
Asia-Pacific Lentiviral Vector Market – Industry Trends and Forecast to 2028
Europe Lentiviral Vector Market – Industry Trends and Forecast to 2028
Middle East and Africa Lentiviral Vector Market – Industry Trends and Forecast to 2028
North America Lentiviral Vector Market – Industry Trends and Forecast to 2028
About Data Bridge Market Research, Private Ltd
Data Bridge Market Research Pvt Ltd is a multinational management consulting firm with offices in India and Canada. As an innovative and neoteric market analysis and advisory company with unmatched durability level and advanced approaches. We are committed to uncover the best consumer prospects and to foster useful knowledge for your company to succeed in the market.
Data Bridge Market Research is a result of sheer wisdom and practice that was conceived and built-in Pune in the year 2015. The company came into existence from the healthcare department with far fewer employees intending to cover the whole market while providing the best class analysis. Later, the company widened its departments, as well as expands their reach by opening a new office in Gurugram location in the year 2018, where a team of highly qualified personnel joins hands for the growth of the company. “Even in the tough times of COVID-19 where the Virus slowed down everything around the world, the dedicated Team of Data Bridge Market Research worked round the clock to provide quality and support to our client base, which also tells about the excellence in our sleeve.”
Contact:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475