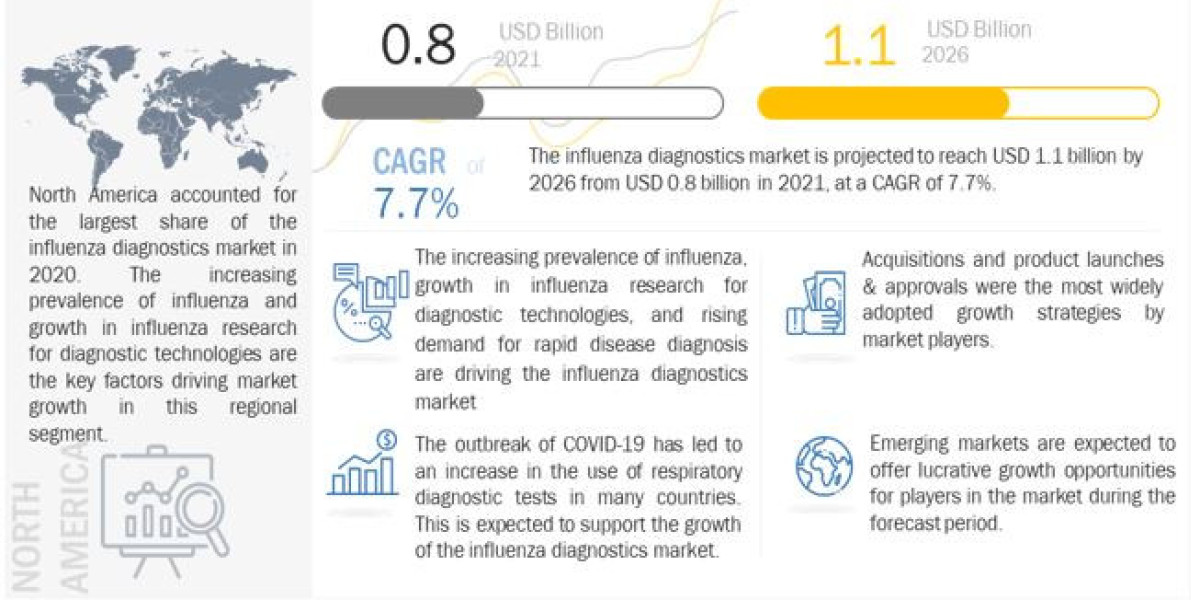
The report "Influenza Diagnostics Market in terms of revenue was estimated to be worth $0.8 billion in 2021 and is poised to grow at a CAGR of 7.7% from 2021 to 2026.
The new edition of the report provides the market size and forecast for each region along with their respective countries. The new edition of the report includes the product segmentation of the market. Market growth is driven by rising demand for rapid disease diagnosis, the increasing prevalence of influenza, and growth in influenza research for diagnostic technologies. Recent developments in this field have led to significant improvements in the global market that have enabled the increased use of influenza diagnostic tests. Influenza diagnostics has also advanced in terms of the variety of tests available, including viral culture tests, serology tests, rapid antigen testing (RT-PCR), and others. Influenza diagnostic tests, earlier used by large hospitals and diagnostic centers, have now become a potentially transformative diagnostic tool for influenza even for small-scale clinics and are also used by the research industries. This shift is primarily driven by rapid improvements in diagnostic technologies.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=222985562
Browse in-depth TOC on "Influenza Diagnostics Market”
154 – Tables
41 – Figures
193 – Pages
Key Market
The major players operating in this market are Danaher Corporation (US), Siemens Healthineers (Germany), Thermo Fisher Scientific, Inc. (US), F. Hoffmann-LA Roche AG (Switzerland), Abbott Laboratories, Inc. (Us), Hologic, Inc. (US), bioMérieux SA (France), Quidel Corporation (US), Becton, Dickinson and Company (US), Meridian Bioscience (US), GenMark Diagnostics, Inc. (US), Luminex Corporation (US), Tecanclinica Trading AG (Switzerland), DiaSorin SA (Italy), altona Diagnostics GmbH (Germany), SEKISUI Diagnostics (US), SA Scientific Ltd. (US), Coris BioConcept SPRL (Belgium), ELITech Group (France), Mast Group Ltd. (UK), Genome Diagnostics, Pvt. Ltd. (India), Germaine Laboratories, Inc. (US), Response Biomedical Corp. (Canada). Tauns Laboratories, Inc. (Japan) and 3B BlackBio Biotech India Ltd. (India).
Test kits and reagents segment accounted for the largest share of the influenza diagnostics market, by product
The market is segmented, based on product, into test kits and reagents, instruments, and other products based on product. In 2020, the test kits and reagents segment accounted for the largest share of this market, mainly due to the increasing prevalence of influenza and rising demand for rapid disease diagnosis.
Molecular diagnostic tests segment accounted for the largest share of the influenza diagnostics market, by test type
Based on test type, the market is divided into molecular diagnostic tests and traditional diagnostic tests. In 2020, the molecular diagnostic tests segment accounted for the largest share. Factors such as growth in influenza research for diagnostic technologies and the increasing prevalence of influenza drive this market.
Diagnostic laboratories segment accounted for the largest share in the influenza diagnostics market, by end users
The market has been segmented based on end users into diagnostic laboratories, hospitals and clinics, and other end users. In 2020, the diagnostic laboratories segment accounted for the largest share of this market. The increasing prevalence of influenza and rising demand for rapid disease diagnosis are driving the growth of this segment.
Driver: Growth in influenza research for diagnostic technologies
The rising prevalence of influenza across the globe has increased R&D efforts towards its effective detection and diagnosis. Most research activity focuses on developing faster and more accurate diagnostic solutions for influenza viruses leading to market growth. National Institute of Allergy and Infectious Diseases (NIAID), a part of the US NIH, initiated the Collaborative Influenza Vaccine Innovation Centers (CIVICs) program to support a broad portfolio of influenza research activities. NIAID announced funding of USD 51 million for CIVICs.
Opportunities: Advancements in genomic and proteomic technologies
The Human Genome Project and advances in molecular and biomedical technologies have generated a vast amount of data, which has resulted in the development of a multitude of assays and technologies useful for the diagnosis and management of influenza infections. These new technologies, based on genomic techniques (such as PCR-based) and proteomics (such as microarray-based detection), help discover new influenza viruses. They also enable better surveillance and rapid diagnosis of infectious diseases, which serves as an opportunity for the market.
Restraints: Variabilities in test sensitivity and specificity
Sensitivity and specificity are the major factors that impact the results of influenza diagnostic tests. The antigenic variation of influenza viruses is the main reason behind the complexity of these tests. As a result, there are variabilities in the sensitivity and specificity of influenza diagnostic tests, which can impact the final test results. Due to this factor, false-negative results are more common than false-positive results, especially during peak influenza season, which is a major factor restraining the growth of the influenza diagnostics market. Recently, the FDA cleared rapid influenza diagnostic tests (RIDTs).
Challenges: Stringent regulatory framework
A major challenge that most diagnostic companies face in commercialising their tests is getting Medicare and private health insurers to pay for them. This is important not only to help the decision-making process of physicians within the practise of evidence-based medicine but also to achieve regulatory approvals and reimbursements for the tests. Regulatory approvals for influenza tests have become more stringent in recent years to ensure their efficiency in detecting all types of influenza viruses.
Request 10% Customization: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=222985562
North America is the largest regional market for influenza diagnostics market
The global influenza diagnostics market is segmented into five major regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2020, North America accounted for the largest share of the influenza diagnostics market. The large share of this region can be attributed to growth in influenza research for diagnostic technologies and rising demand for rapid disease diagnosis.
Report Link: Influenza Diagnostics Market
Recent Developments:
- In May 2021 Becton, Dickinson and Company (US) received FDA approval for the BD Veritor Plus System, which is used to detect SARS-CoV-2, influenza A, and influenza B.
- In March 2021, Abbott Laboratories, Inc. (US) received emergency use authorization (EUA) from the FDA for a laboratory PCR assay that detects and differentiates SARS-COV-2, flu A, flu B, and RSV in one test.
- In February 2021, Becton, Dickinson and Company (US) received approval from the FDA for the emergency use authorization (EUA) for a new molecular diagnostic test for both SARS-CoV-2 and influenza A+B.
Table of Content:
1 INTRODUCTION (Page No. - 28)
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- CURRENCY
- LIMITATIONS
- STAKEHOLDERS
- SUMMARY OF CHANGES
- RESEARCH METHODOLOGY (Page No. - 34)
- RESEARCH DATA
- RESEARCH APPROACH
- MARKET SIZE ESTIMATION
- MARKET BREAKDOWN & DATA TRIANGULATION
- EXECUTIVE SUMMARY (Page No. - 47)
- PREMIUM INSIGHTS (Page No. - 50)
- MARKET OVERVIEW (Page No. - 53)
- INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT (Page No. - 70)
- INFLUENZA DIAGNOSTICS MARKET, BY TEST TYPE (Page No. - 76)
- INFLUENZA DIAGNOSTICS MARKET, BY END USER (Page No. - 89)
- INFLUENZA DIAGNOSTICS MARKET, BY REGION (Page No. - 93)
- INTRODUCTION
- NORTH AMERICA
- EUROPE
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their pain points around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 Micro Quadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, "Knowledge Store" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Research Insight: https://www.marketsandmarkets.com/ResearchInsight/influenza-diagnostic-market.asp
Visit Our Website: https://www.marketsandmarkets.com/
Content Source: https://www.marketsandmarkets.com/PressReleases/influenza-diagnostic.asp