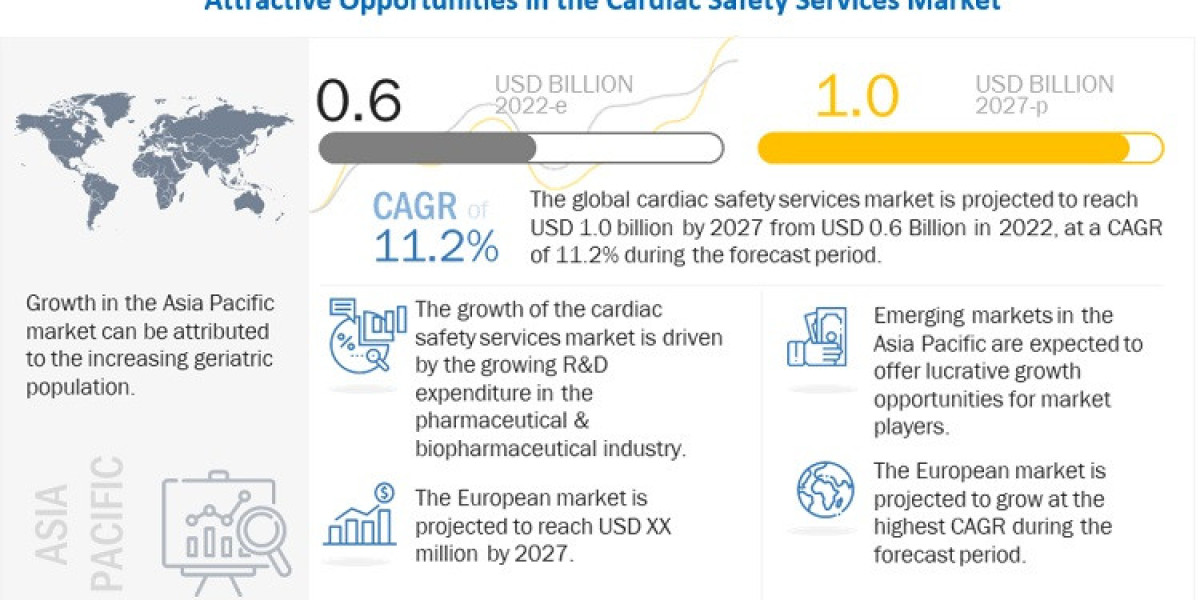
The Global Cardiac Safety Services Market is expected to experience significant growth over the forecast period, which spans from 2022 to 2027. The market is projected to reach USD 1.0 billion by 2027, exhibiting a Compound Annual Growth Rate (CAGR) of 11.2%. This growth can be attributed to several factors.
One of the key drivers of the market growth is the increasing research and development (R&D) expenditure in the pharmaceutical and biopharmaceutical industry. As these industries invest more in the development of new drugs and therapies, the demand for cardiac safety services, which play a crucial role in ensuring the safety and efficacy of these products, is likely to rise.
Moreover, the outsourcing of R&D activities by pharmaceutical companies is contributing to the expansion of the cardiac safety services market. Many pharmaceutical firms are opting to outsource certain aspects of their R&D processes to specialized service providers, including cardiac safety services. This allows them to focus on their core competencies while benefiting from the expertise and efficiency of external service providers.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=174488627
Browse in-depth TOC on "Cardiac Safety Services Market”
113 – Tables
28 – Figures
132 – Pages
Key Market Players
The prominent players operating in this market include Laboratory Corporation of America Holdings (US), Koninklijke Philips N.V. (Netherlands), Clario (US), Banook Group (France), IQVIA (US), Biotrial (France), Certara (US), Celerion (US), Medpace (US), Ncardia (Netherlands), Richmond Pharmacology (UK), PhysioStim (France), Shanghai Medicilon (China), Pharmaceutical Product Development (US), and SGS (Switzerland). Expansion and acquisitions are the key growth strategies undertaken by these companies to maintain their positions in the market.
Drivers:
- Rising R&D Expenditure in Pharmaceutical & Biopharmaceutical Industry: Pharmaceutical companies are increasing their R&D investments to deliver high-quality and innovative products. This, coupled with collaborative R&D efforts, is leading to a higher demand for preclinical and clinical services, including cardiac safety evaluation.
Opportunities:
- Growth in Biosimilars and Biologics Markets: The development of biologics and biosimilar molecules is on the rise. Pharmaceutical and biopharmaceutical companies are heavily investing in R&D for these products, creating opportunities for safety assessment providers to expand their portfolios and capabilities.
Restraints:
- High Cost of Cardiac Safety Services: Cardiac safety evaluations of off-target drug effects can be expensive and time-consuming. This cost burden can contribute to the termination of many new drug development projects, affecting the demand for cardiac safety evaluation studies and hindering overall drug development.
These factors play a crucial role in shaping the dynamics of the cardiac safety services market. The projected CAGR of 11.2% during the forecast period reflects the potential for growth in this sector, driven by the factors mentioned above. Companies in this industry will need to address the challenges related to high costs and leverage the opportunities presented by the growth in biologics and biosimilars to capitalize on the expanding market demand.
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=174488627
The pharmaceutical & biopharmaceutical accounted for the largest share of the end user segment in the cardiac safety services market in 2021. Pharmaceutical & biopharmaceutical companies primarily use cardiac safety services to manage clinical trials for newly developed drugs. Companies are engaged in the development of novel drugs for the treatment of various diseases. Cardiac safety issues are among the most common reasons promising drugs can be halted during development. As a result, there is a high demand for cardiac safety services among pharmaceutical and biopharmaceutical companies.
Recent Developments
- In February 2021, Koninklijke Philips N.V. (Netherlands) acquired Biotelemetry (US). This acquisition enhanced the company’s cardiac care portfolio and transformed care delivery along the health continuum with integrated solutions.
- In March 2019, BioTelemetry acquired Geneva Healthcare, Inc., a leading provider of remote monitoring for implantable cardiac devices.
Report Link: Cardiac Safety Services Market
The cardiac safety services market is segmented into four major regions—North America, Europe, Asia Pacific, and the Rest of the World (RoW). In 2021, North America commanded the largest share of the global cardiac safety services market, followed by Europe. The large share of the North American market is attributed to factors such as the large pharmaceutical & biopharmaceutical industry in the region, the rising R&D expenditure, stringent regulations, and the presence of major providers of cardiac safety services in the US and Canada.
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their pain points around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 Micro Quadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, "Knowledge Store" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Visit Our Website: https://www.marketsandmarkets.com/
Content Source: https://www.marketsandmarkets.com/PressReleases/cardiac-safety-services.asp