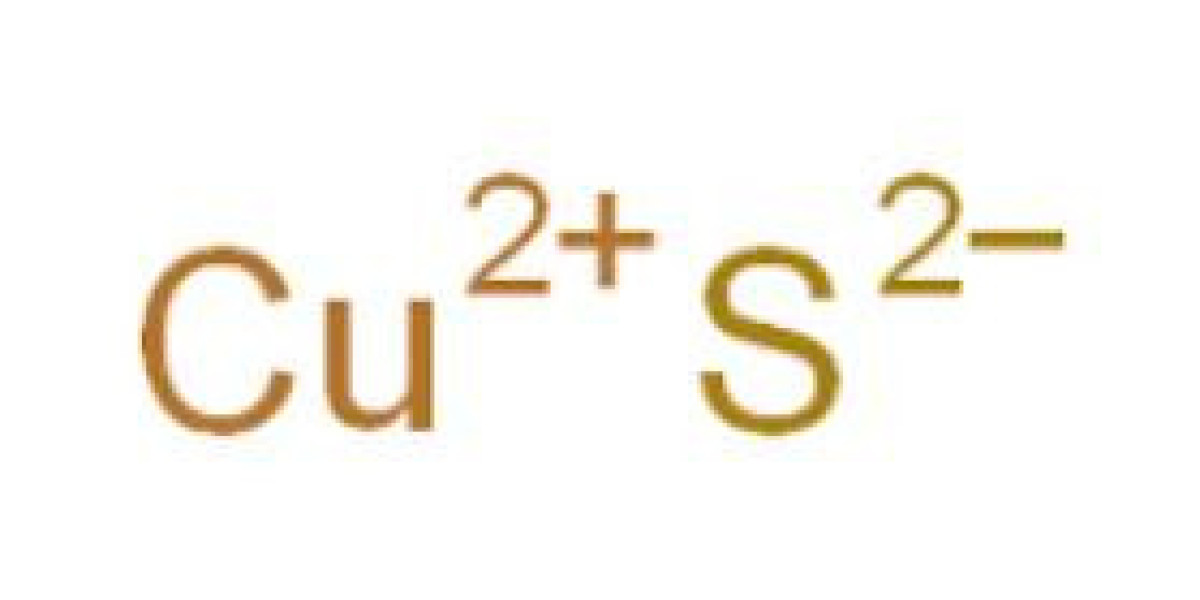Electrocatalytic semi-hydrogenation of alkynes to alkenes using low-cost precious metal-free catalysts with water as the hydrogen source is ideal, but challenging because of their over-hydrogenation to unwanted alkanes. Here, we propose that the ideal catalyst should have appropriate binding energy with active atomic hydrogen (H*) from hydroelectrolysis and weak adsorption with alkenes, thus promoting semi-hydrogenation of alkynes and avoiding over-hydrogenation. Therefore, a low coordination copper nanowire sponge with surface sulfur doping and adsorption has been synthesized by in-situ electroreduction of copper sulfide, and the semi-hydrogenation of alkynes with water as the electrocatalytic source is more than 99% selective, which is superior to copper nanowire without surface sulfur. The sulfur-anion hydrated cation (S2− − K+ (H2O) n) network between S2− and K+ adsorbed on the surface of the KOH electrolyte promotes the generation of hydroelectrolysis activity H*. The doping of trace sulfur weakens the adsorption of olefin and avoids excessive hydrogenation. Our catalyst also shows a wide substrate range, up to 99% olefin selectivity, good reducible group compatibility, and easy synthesis of deuterated olefin, highlighting the good potential of this method.
Selective hydrogenation of alkynes to olefins is an important fundamental transformation in synthetic chemistry. This conversion is also a classical model reaction for evaluating the performance of newly developed catalysts or hydrogenation methods. Although significant progress has been made, current reports still rely primarily on expensive precious metal catalysts (e.g., Pt, Pd, Ru or their related alloys) or complex metal complexes with gaseous hydrogen (H2) or expensive and/or toxic organic hydrogen sources, raising serious concerns about cost, safety and sustainability. To address these issues, our group has recently developed an electrochemical strategy to selectively semi-hydrogenate alkyne 18 on Pd-P cathodes by using H2O as a hydrogen source. However, good olefin selectivity requires precise control of the applied potential and reaction time. Therefore, the extended hydrogenation time or potential sensitivity of alkynes to alkanes remains a major obstacle to the actual synthesis of high purity alkenes. At present, there is still a lack of a catalyst that can essentially control the semi-hydrogenation of alkynes to olefin. Therefore, there is an urgent need to explore a non-precious metal nanostructured electrocatalyst for the semi-hydrogenation of highly selective alkynes with a wide potential for time-independent selectivity.
Electrochemistry provides an efficient and sustainable platform for the synthesis of high value-added fine chemicals. Copper based catalysts are widely used in many electrochemical reactions because of their abundance, robustness and excellent catalytic activity. Due to the relatively large hydrogen adsorption free energy (ΔGH*), Cu materials can inhibit competitive hydrogen development reactions (HER) and are therefore ideal candidates for electroreduction reactions such as carbon dioxide reduction reactions (CO2 RR), deuteration of halides, and nitrate RR. Doping and surface modification favor high carbon products for CO2 reduction by controlling the adsorption of intermediates. In fact, electrode materials often undergo structural reconstruction, including changes in morphology, composition and crystal surface under electrochemical conditions. For example, copper oxide-derived nanostructures exhibit improved performance against CO2 RR in the presence of rich grain boundaries, exposed high exponential crystal faces, and low coordination Cu. Metal chalcogenides and MoNi alloys were reconstructed to produce surface-adsorbed chalcogenides and Mo2O72− ions, both of which have been shown to accelerate hydroelectrolysis. In addition, the adsorption of inert Au or In by Sδ− on the surface promotes hydroelectrolysis to produce active atomic hydrogen (H*). In addition, in the hot catalysis with H2 participation, doping S in Pd can weaken the adsorption of olefin and inhibit its overhydrogenation. Semi-hydrogenation of alkynes to olefin can also be achieved by homogeneous and heterogeneous Cu catalysis at high temperature and under high pressure of H2. In these respects, we speculate that sulfur-modified Cu nanostructures, especially those synthesized by in-situ electroreduction of self-supported precursors, may be very effective for electrocatalytic semi-hydrogenation of alkynes. However, Cu nanostructures with surface sulfur formed by in-situ electrochemistry for selective semi-hydrogenation of alkynes using H2O as a hydrogen source have not been explored.
In this paper, Cu nanowire sponges (CU-S-Ns) doped and adsorbed with sulfur surface were designed and synthesized by in-situ electroreduction of copper ii sulfide nanowire array /Cu foam (CUS-NAS). Using H2O as hydrogen source, Cu-S NSs has high activity, selectivity up to 99%, wide substrate range and good functional group compatibility for semi-hydrogenation of alkynes. Electrochemical in situ X-ray diffraction (XRD), Raman spectroscopy and X-ray absorption spectroscopy (XAS) have demonstrated the transformation process from CuS-NAs to Cu-S-NSs. Both experimental and computational results reveal the key role of surface adsorbed and lattice-doped sulfur in the selective semi-hydrogenation of alkynes in Cu-S NSs. This sulfur-promoted method is also suitable for hydrodehalogenation of aryl iodine.