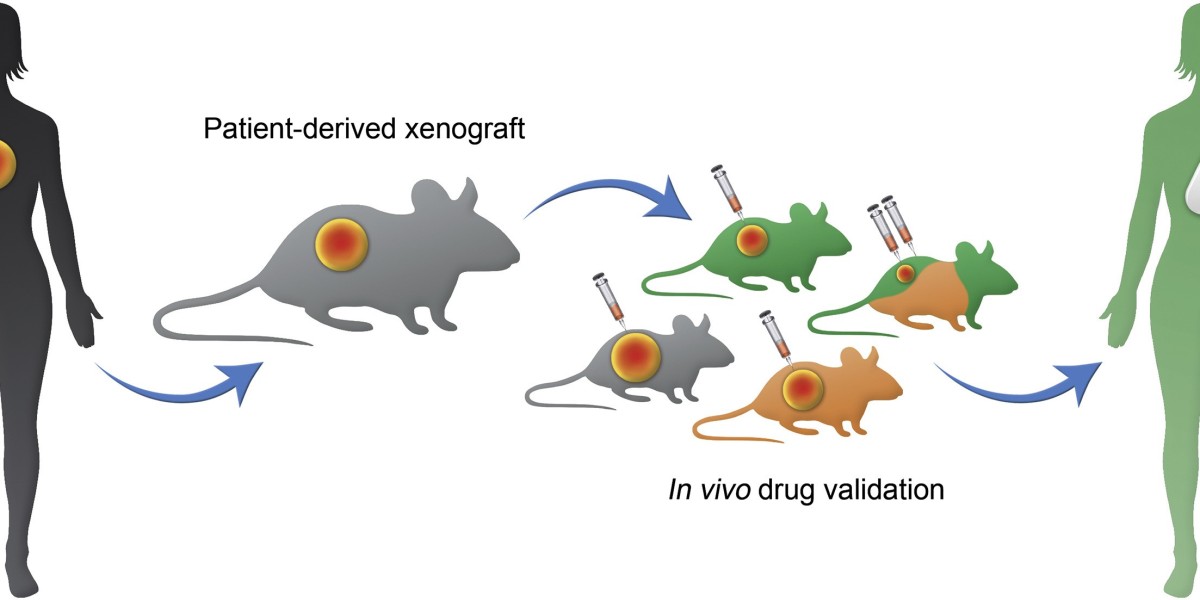
The landscape of cancer research has evolved remarkably over the past few decades, with various models developed to study tumor biology and test potential treatments. Among these, Patient-Derived Xenograft (PDX) models have emerged as a powerful tool, bridging the gap between traditional cell lines and clinical studies. By implanting human tumor tissues into immunodeficient mice, PDX models maintain the histological and genetic characteristics of the original patient’s tumor, offering a more accurate and clinically relevant representation of cancer.
Historical Background
The concept of Patient-Derived Xenograft Models dates back to the 1960s when scientists began implanting human tumors into mice to study cancer biology. However, the significant breakthrough came with the development of immunodeficient mouse strains, such as nude and SCID (severe combined immunodeficiency) mice, which lack functional T-cells and do not reject human tissues. This advancement allowed for the successful engraftment and growth of human tumors in a mouse model, leading to the rise of PDX models.
Methodology
The process of creating a PDX model involves several steps. First, a tumor sample is obtained from a patient through surgical resection or biopsy. This sample is then implanted into the subcutaneous or orthotopic site of an immunodeficient mouse. Once the tumor establishes and grows in the mouse, it can be re-transplanted into additional mice to create a cohort for experimental studies. This iterative process helps in maintaining the tumor’s heterogeneity and genetic fidelity over multiple generations.
Advantages of PDX Models
PDX models offer numerous advantages over traditional cell lines and genetically engineered mouse models. One of the primary benefits is the preservation of the tumor microenvironment, including stromal and immune components, which play a crucial role in tumor growth and response to therapy. Additionally, PDX models retain the genetic and histological complexity of the patient’s tumor, providing a more accurate platform for studying tumor behavior and drug response.
Applications in Cancer Research
PDX models are extensively used in various aspects of cancer research. They serve as invaluable tools for studying tumor biology, understanding mechanisms of drug resistance, and identifying potential biomarkers for personalized medicine. Moreover, PDX models are instrumental in preclinical drug evaluation, allowing researchers to test the efficacy and toxicity of new therapeutic agents in a clinically relevant setting. This has led to the identification of promising compounds that have progressed to clinical trials.
Challenges and Future Directions
Despite their advantages, PDX models are not without limitations. The use of immunodeficient mice limits the ability to study immune-oncology therapies, which are increasingly important in cancer treatment. Additionally, maintaining and expanding PDX models can be costly and time-consuming. Future advancements aim to address these challenges by developing humanized PDX models that incorporate human immune cells, thus allowing for the study of immune response and immunotherapies. Moreover, efforts are underway to reduce the cost and improve the scalability of PDX models through advanced technologies such as 3D bioprinting and organ-on-chip systems.
Patient-Derived Xenograft models have revolutionized cancer research, providing a robust and clinically relevant platform for studying tumor biology and testing new therapies. While challenges remain, ongoing advancements promise to further enhance the utility of PDX models, paving the way for more effective and personalized cancer treatments.
Get More Insights On- Patient Derived Xenograft Model