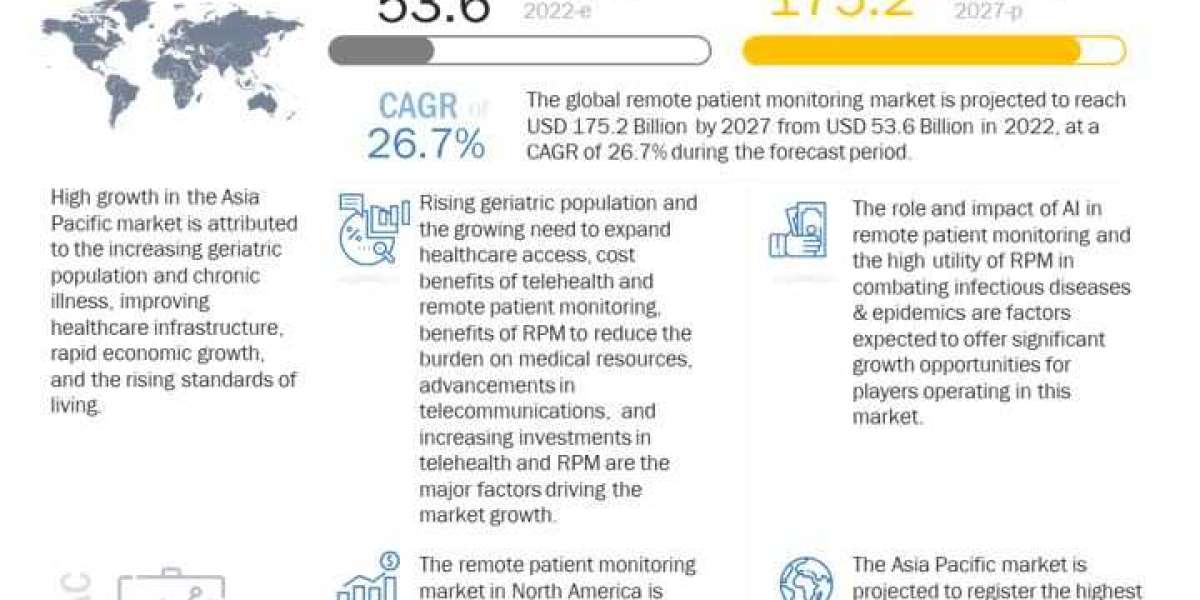
[208 Pages Report] The global bioanalytical testing services market is projected to reach USD 6.0 billion by 2027 from an estimated USD 2.9 billion in 2022, at a CAGR of 15.6% during the forecast period. In 2021, North America accounted for the largest share of the market, followed by Europe.
The major factors influencing the growth of the North American market include the well-established pharmaceutical industry, strong presence of major service providers, large number of ongoing clinical trial studies, high RD expenditure, growth in the biosimilars and generics markets, and the rise in the outsourcing of preclinical, clinical, and laboratory testing services by pharmaceutical and biopharmaceutical companies in the region.
The COVID-19 pandemic has led to a global healthcare crisis, resulting in widespread economic impact since January 2020. In the optimistic scenario, it could be assumed that the COVID-19 pandemic would create a long-term positive impact on the overall market.
The market was initially negatively impacted by the COVID-19 pandemic. Along with the rest of the world, the pharmaceutical companies shut down their laboratories and industry work was stopped in order to mitigate the pandemic effects. Furthermore, the on-site personnel restrictions had biggest impact on pharmaceutical businesses. Most of the large Pharma companies were completely shut down momentarily.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=12254971
Additionally, the clinical sites also experienced reduced enrolment and restricted staff. These challenges combined to reduce anticipated trial sizes and created substantial delays in study timelines. Though, recovery can be seen in most regions, particularly North America and Europe, as services regain normality.
The Asia Pacific market has been sluggish to recover, notably in China and India. In an optimistic scenario, the need for smoother workflows and faster turnaround times could boost the market growth.
Biologics represent one of the most promising new therapeutic areas and are becoming increasingly important in the pharmaceutical market; around 800 products are in the pipeline at present. One of the key reasons for this is the rising prevalence of chronic diseases. However, considering the high cost of biologics and the rising focus of various governments on reducing healthcare costs, the focus on biosimilars has increased in recent years.
Currently, many pharmaceutical and biopharmaceutical companies are focusing on building a diverse product portfolio and developing new small and large molecules. Combination products, delivery devices, and reformulated or re-engineered drugs are being developed by companies to cater to the unmet needs in the market.
Key players in the Bioanalytical testing services market: Charles River (US), Medpace (US), WuXi AppTec (China), Eurofins Scientific (Luxembourg), IQVIA (US), SGS SA (Switzerland), Laboratory Corporation of America Holdings (US), Intertek Group (UK), Syneos Health (US), ICON (Ireland), Frontage Labs (US), PPD (US), PAREXEL International Corporation (US), Almac Group (UK), Celerion (US), Altasciences (US), BioAgilytix Labs (US), Lotus Labs (India), LGS Limited (UK), Sartorius AG (Germany), CD BioSciences (US), Absorption Systems LLC (US), Pace Analytical Services (US), Bioneeds India Private Limited (India) and Vipragen Biosciences (India).