Clinical trials are research studies conducted on humans to evaluate the safety and efficacy of new drugs, treatments, or medical procedures. They are conducted by medical professionals and researchers to collect data about the safety and effectiveness of a potential treatment or medical device. Clinical trials are a critical part of the process of bringing new treatments and therapies to market.
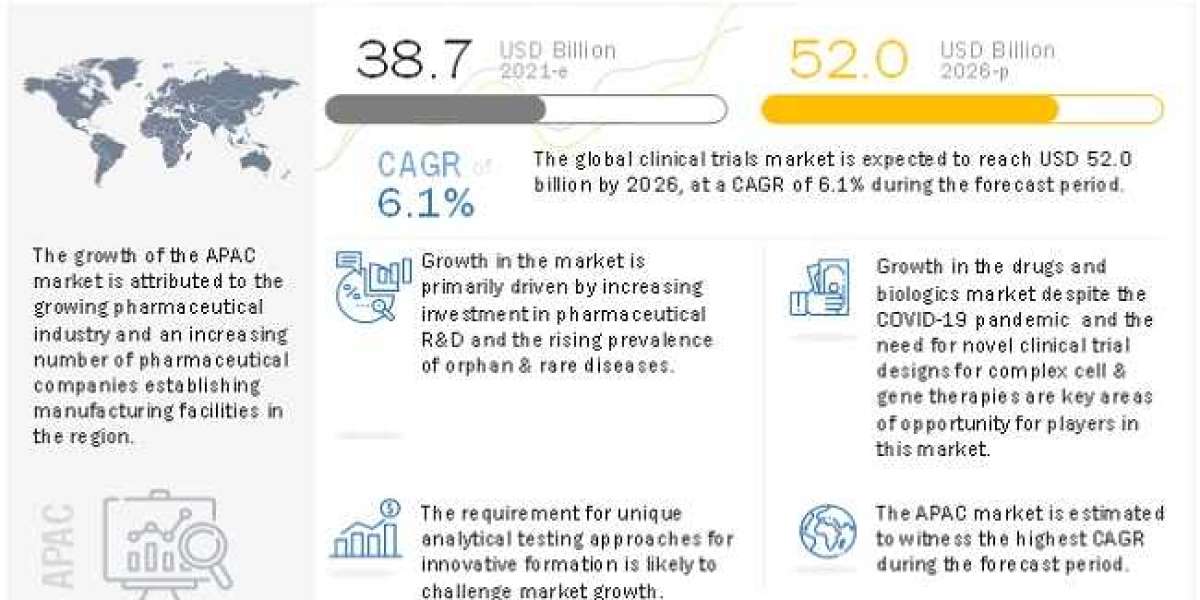
Currently, Clinical Trials Market is projected to reach USD 52.0 billion by 2026 from USD 38.7 billion in 2021, at a CAGR of 6.1% during the forecast period.
Phase III segment accounted for the largest share of clinical trials market
Based on phase, the clinical trials market is segmented into phases I, II, III, and IV. Phase III dominated the market in 2020. Services that incur high expenses and large patient/population size are the key factors attributing to the high share of the Phase III segment.
Request for assumptions how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=405
Market Growth Drivers
- Increasing investments in pharmaceutical Ramp;D
- Increasing number of clinical trials
- High cost of in-house drug development
Growth Opportunities
- Growth in drugs and biologics market
- Rising demand for specialized testing services among end users
- Need for novel clinical trial designs for complex cell and gene therapies
The prominent players operating in the clinical trials market are IQVIA (US), LabCorp (US), PPD (US), PRA Health Sciences (US), Syneos Health (US), Charles River Laboratories (US), WuXi AppTec (China), Paraxel International (US), and ICON Plc (US).
IQVIA (US) dominates the clinical trials market. This is mainly due to its strong performance in the biopharmaceutical services industry and wide geographical coverage. The company’s foothold in the market is primarily attributed to various factors such as strong technical service capabilities, good client relationships, diversified service offerings, and its ability to expand the penetration of its offerings. The company has registered significant growth in the biopharmaceutical services industry. It is present in all major markets, including the US, Japan, Germany, France, Spain, and Italy, in addition to BRIC. To increase its geographic presence and customer base, the company focuses on service launches, collaborations, and expansions.
Syneos Health (US) is one of the top 3 key players in the global clinical trials market. A diverse range of services are included under the Clinical Solutions business segment of Syneos Health that spans Phases I to IV of clinical development, which has strengthened the companys market position in 2020. Additionally, amidst the COVID-19 associated challenges, the company focuses on expanding its decentralized clinical trial capabilities rendering Syneos Health a strong foothold in the clinical trials market. In June 2021, the company announced the inclusion of a dedicated Decentralized Clinical Trials Site Advocacy Group, encouraging the adoption of decentralized solutions among professionals.
What Are the Benefits of Participating in a Clinical Trial?
Participating in a clinical trial can provide several benefits to those involved. Not only can participants gain access to new treatments and medications before they become available to the general public, but they can also play an important role in advancing medical innovation. Participants also have the chance to receive personalized medical care and support, as well as financial compensation for their time and effort.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=405
Recent Developments
- In September 2021, Syneos Health entered into a strategic collaboration with Ride Health to offer non-emergency medical transportation (NEMT) for clinical trial participants.
- In April 2021, IQVIA acquired Q2 Solutions, a clinical laboratory services organization, from Quest Diagnostics.
- In November 2020, WuXi Apptec expanded its Cell amp; Gene Therapy Platforms with capabilities to provide high-quality and cost-effective supplies of RD and GMP Plasmids
- In April 2020, IQVIA launched COVID-19 trial matching service to accelerate treatment and vaccine development against the COVID-19 pandemic in U.S. The company launched comprehensive online screener and trial matching tool for all COVID-19 trials in the US.
- In May 2020, IQVIA announced the Japan and Asia Pacific expansion of IQVIA Biotech to deliver integrated clinical solutions and support biotech and emerging biopharma companies.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/clinical-trials-market-405.html