Virtual Clinical Trials Market Highlights
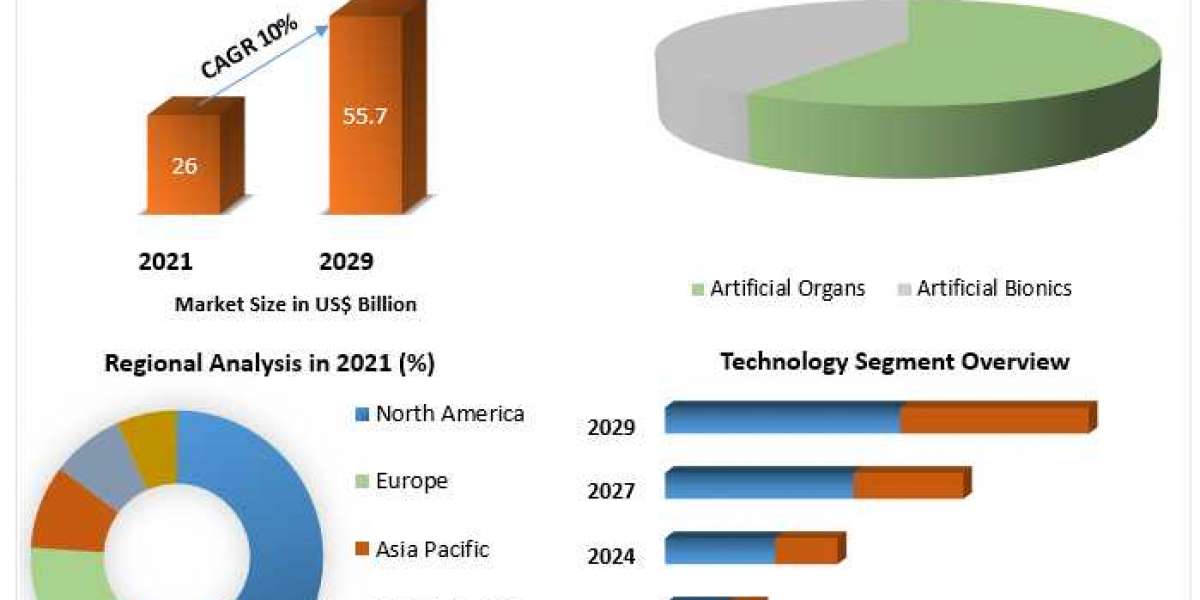
According to MRFR analysis, the global virtual clinical trials market size is expected to register a CAGR of ~5.89% during the forecast period of 2021 to 2027 and is expected to reach ~USD 11,239.0 million by 2027.
Virtual clinical trials are methods of conducting clinical research using apps, electronically monitoring devices and online social engagement platforms. In 2020, 76% of more than 200 clinical trial sponsors said they conducted most or all of their patient monitoring remotely, up from only 18% of respondents the previous year, according to the 2021 State of the Industry Report from Florence Healthcare (US), a provider of digital research workflows and remote site access. This exponential increase in number of clinical trials monitored digitally is expected to increase the growth of the global virtual clinical trials market during the forecast period.
The global virtual clinical trials market is currently dominated by several market players. The key players are involved in geographic expansion, acquisition, and strategic collaborations to maintain their global footprint in the global virtual clinical trials market. For instance, in June 2020, Tabula Rasa HealthCare (US), a New Jersey-based company collaborated with Washington-based health plan provider Regence (US) to virtually trial several different COVID-19 drug candidates and assess the risk of adverse drug events. Regence will provide de-identified medical information from about 500,000 of its members to Tabula Rasa HealthCare. Thus, an increasing number of healthcare companies collaborating with technology companies to leverage data-driven clinical studies for development of effective medication by monitoring trials remotely is increasing global virtual clinical trials market size. The demand created by pandemic for adoption of monitoring trials remotely is expected to increase the lucrativeness of virtual clinical trials market during the forecast period.
Regional Analysis
The market has been divided, by region, into the North America, Europe, Asia-Pacific, and Rest of the World.
North America is anticipated to hold the largest market share owing to availability of major players operating in the region. Medable, Inc. (US), Oracle Corporation (US), IQVIA Holdings, Inc. (US), Syneos Health (US), PPD, Inc. (US) and Medpace Holdings, Inc. (US) are some of the notable companies operating in the region. Moreover, collaborative approach by companies operating in the region were observed due to rising demand and continuation of early phase clinical studying during the pandemic. For instance, during October 2020, FHI Clinical Inc. (US), a contract research organization adopted Oracle’s (US) Oracle Health Sciences Clinical One, a cloud platform for effective study management throughout the entire drug development lifecycle. FHI clinical also uses Oracle Argus Safety for safety case management; Oracle Clinical Trial Management System Cloud Service to manage the research portfolio; and Oracle ClearTrial Cloud Service to manage projects. Thus, increasing adoption of cloud-based platforms by contract research organizations operating in the region are anticipated to fuel the growth of virtual clinical trials market. The virtual clinical trials market in North America is divided into the US and Canada.
European virtual virtual clinical trials market outlook has been categorized into Germany, France, the UK, Italy, Spain, and the rest of Europe. Europe is anticipated to hold the significant market share owing to investment activities related to clinical trials in the region and support from government for RD. For example, Parexel International Corporation (US) in November 2020 announced a strategic partnership between its Early Phase Clinical Unit (Germany) and Clinical Trial Center (CTC) North, a full-service CRO located at the University Medical Center Hamburg-Eppendorf (Germany). Parexel intends to roll-out and implement eSource system ClinBase at CTC North to drive efficiency. An increasing number of research institutes partnering with leading clinical trial services provider to accelerate development of products for different conditions such as cancer, COVID-19 and other disorders is expected to fuel growth of virtual clinical trials market during the forecast period. Moreover, ongoing research studies being converted to virtual mode are expected to increase the demand for IT services, thereby generating opportunities for IT companies to access virtual clinical trials market. Thus, long-term partnerships among research institutes and service providers to accelerate ongoing research studies is anticipated to increase the lucrativeness of virtual clinical trials market in the region during the forecast period.
The virtual clinical trials market in Asia-Pacific has been segmented into Japan, China, India, South Korea, Australia, and the rest of Asia-Pacific. The growth of virtual clinical trials market in Asia-Pacific is anticipated to witness rapid growth during the forecast period owing to increasing number of memorandum signed by governmental agencies to promote research activities. For example, India and the UK announced a new memorandum of understanding in November 2020 between the Central Drugs Standard Control Organisation (India) and the UK Medicines and Healthcare Products Regulatory Agency. This memorandum is expected to increase bilateral regulations, sharing of information to control the trade of unlicensed products and 10-year roadmap for bilateral cooperation on the COVID-19 vaccine. Moreover, during April 2020 the Chinese government issued a plan of action in order to promote virtual clinical trials. Apart from support from governmental agencies to promote decentralized clinical trials, pharmaceutical companies operating in the Asia-Pacific are adopting to report clinical trials data virtually. For instance, on October 21, 2021 Shionogi Co., Ltd (Japan) presented results of its investigational oral antiviral drug for COVID-19 at the International Society for Influenza and Other Respiratory Virus Diseases, World Health Organization Virtual Conference. Thus, increasing support for RD through multilateral memorandum of understanding among government bodies and need to present data virtually is expected to propel the growth of virtual clinical trials market in the region.
The virtual clinical trials market in Rest of the World has been divided into the Middle East, Africa and Latin America. Africa contributed to less than 2% of the total number of clinical trials during 2020. The African Academy of Sciences (AAS) launched the Clinical Trials Community online platform during November 2020. The launch of such online platform promotes the progression of intra-African collaboration around clinical trials.
Segmentation
The global virtual clinical trials market has been segmented based on study type, phase, and indication.
The market, based on study type, has been divided into interventional, observational and others. The interventional segment is likely to hold maximum market share in the global virtual clinical trials market owing to its larger utility and recruitment of patients for testing novel drugs.
The virtual clinical trials market based on phase has been segmented into Phase 1, Phase 2, Phase 3 and Phase 4. The Phase 1 segment is likely to hold significant share in the market as every clinical trial study proceeds through Phase 1.
The virtual clinical trials market based on indication has been segmented into oncology, cardiovascular, immunology, gastrointestinal, respiratory, endocrinology, ophthalmology and others. The oncology segment is likely to hold the largest share in the market due to larger number of clinical trials focused on finding treatment options for cancer.
Key Players
Some of the key players in the global virtual clinical trials market are Oracle Corporation (US), IQVIA Holdings, Inc. (US), Dassault Systemes SE (France), Medpace Holdings, Inc. (US), Icon plc (Ireland), Laboratory Corporation of America Holdings (US), Parexel International Corporation (US), Medable, Inc. (US), Clinical Ink, Inc. (US), Wuxi AppTech (China), and Medable, Inc. (US) among others.
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Consulting Services. MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients.
Contact us:
Market Research Future (part of Wantstats Research and Media Private Limited),
99 Hudson Street, 5Th Floor,
New York, New York 10013
United States of America
+1 628 258 0071
Email: [email protected]