Cuprous oxide is also commonly known as copper oxide which is basically an inorganic compound comprising of copper and oxygen. It has some excellent properties that enable it to surpass a lot of copper compounds. They have semiconducting properties as well which enable them to possess their related applications.
There are a lot of applications of cuprous oxide as there are several different types and ways in which cuprous oxide exists. All these different types are produced after going through various processes which are all authentic in their nature. Their capabilities are all dependent upon the properties that these compounds exhibit and eventually lead up to the different applications, all of which are highly unique in their nature.
Introduction
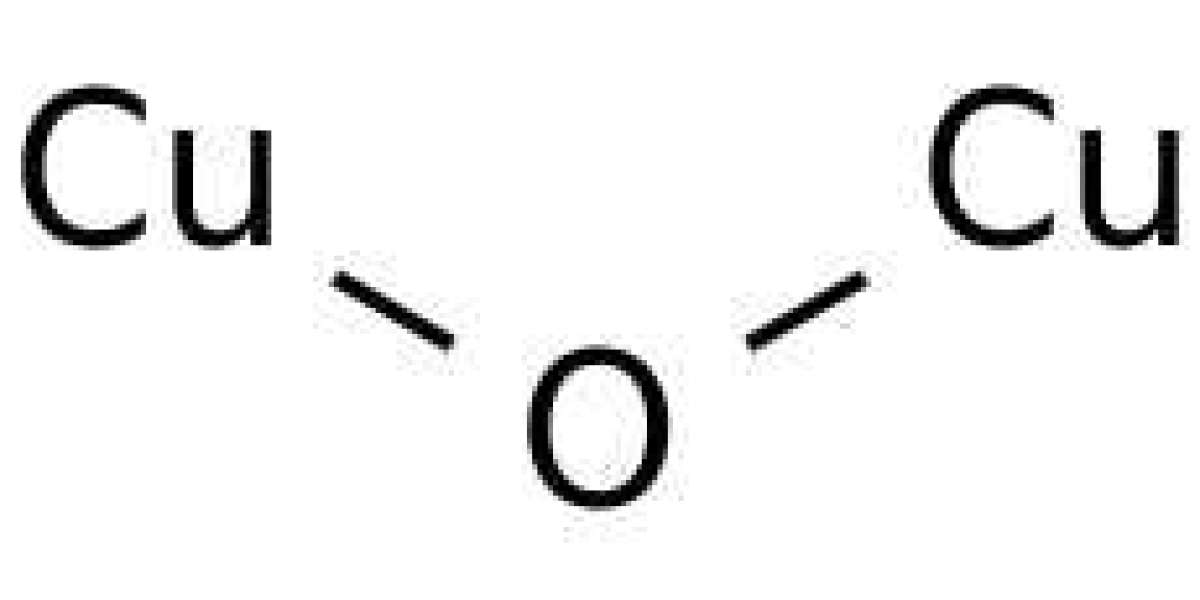
With Cu2O as its formula, Cuprous oxide or Copper (I) oxide is the inorganic compound. One of copper’s principal oxides is known as copper (I) oxide or cuprous oxide, the other being cupric oxide (CuO) or copper (II) oxide. It is a solid red color and it is a component of some of the antifouling paints. The color of this compound can be red or yellow, it is determined by the particle's size. One can find copper(I) oxide as cuprite that is a reddish mineral.
Properties of Cu2O
It is a diamagnetic solid. The oxides are tetrahedral and the copper centers are 2-coordinated in terms of their coordination spheres. In a way, there is a resemblance of the structure with SiO2’s main polymorphs, and interpenetrated lattices were featured by both of the structures. Colorless complex [Cu(NH3)2]+ is formed by dissolving copper(I) oxide in concentrated ammonia solution and that colorless complex easily changes to the blue [Cu(NH3)4(H2O)2]2+, after being oxidized in the air. It gives CuCl-2 solutions by dissolving in hydrochloric acid. Copper (II) nitrate and copper (III) sulfate and produced by diluted nitric acid and sulfuric acid. In moist air, Cu2O degrades to the copper (II) oxide.
Cu2O's lowest excitons are very long-lived. neV linewidths are used to explain the absorption lineshapes, which is the narrowest bulk exciton resonance that has ever been observed. Low group velocity is possessed by the associated quadrupole polaritons and they approach the sound’s speed. Therefore, in this medium, the movement of light is as slow as sound, leading to high polariton densities. Ground state excitons have many remarkable features and one of them is that all of the primary scattering mechanisms are quantitatively known. Cu2O was a substance where a completely parameter-free model of absorption linewidth widening by temperature can be established, enabling the deduction of the corresponding absorption coefficient. Kramers-Kronig relations don't apply to the polaritons and Cu2O can be used to show it.
Basic Strategies to Synthesize Faceted Cu2O Crystals
Many synthetic methods like irradiation technique, sputtering, electrodeposition, and wet-chemistry route like solvothermal synthesis, hydrothermal synthesis, and liquid reduction, can be used to prepare faceted Cu2O micro-/nanocrystals. The most broadly utilized method among those is the wet-chemistry method for manipulating Cu2O crystals’ exposure facets due to the versatile ability in the tailoring of the growth rates and nucleation along various orientations.
Gibbs-Wulff‘s law theoretically determines the crystal’s equilibrium shape. Facets of high surface energies will generally reduce from the final appearance or disappear under equilibrium conditions particularly for the high-index facets. However, under realistic conditions, the interplay between kinetics and thermodynamics results in the exposed facets and the final shapes of crystals.
Thermodynamic Viewpoint
According to a thermodynamic viewpoint, the inherent need to lessen the total surface energy drives the crystal’s shape-evolution during its growth process. Capping reagent’s specific facet-selective adsorption (including inorganic ion, impurity molecule, polymer, and surfactant) in a solution-phase system is an efficient method to expose various facets and reduce surface energy, leading to the appearance of a non-equilibrium Wulff construction. Capping reagent’s role in tailoring the crystal’s morphology offers a guideline for rational design and synthesizing of the Cu2O micro-/nanocrystals with required surface characteristics.
Capping Agent’s Capability and Selectivity
Basically, the distance between two adjacent undercoordinated Cu atoms on the facets and/or the density of undercoordinated Cu atoms controls the Capping agent’s capability and selectivity on various facets for a Cu2O crystal. Thus the capping agent’s choice is important in controlling the Cu2O crystals’ preserved facets. It is due to the diversities of the organic capping agents that they perform a major role in controlling Cu2O crystals shapes. For example, sodium dodecyl sulfate (SDS) and poly(vinyl pyrrolidone) (PVP) with various charges, can function as the capping agents of facets.