The Growing Pharmaceutical Contract Manufacturing Market 2021-2026: Opportunities and Challenges
The global pharmaceutical contract manufacturing market is projected to grow at a healthy rate over the forecast period. This is due to the rising demand for contract manufacturing services from the pharmaceutical and biotechnology industries, as well as the cost savings associated with outsourcing production to contract manufacturing organizations (CMOs).
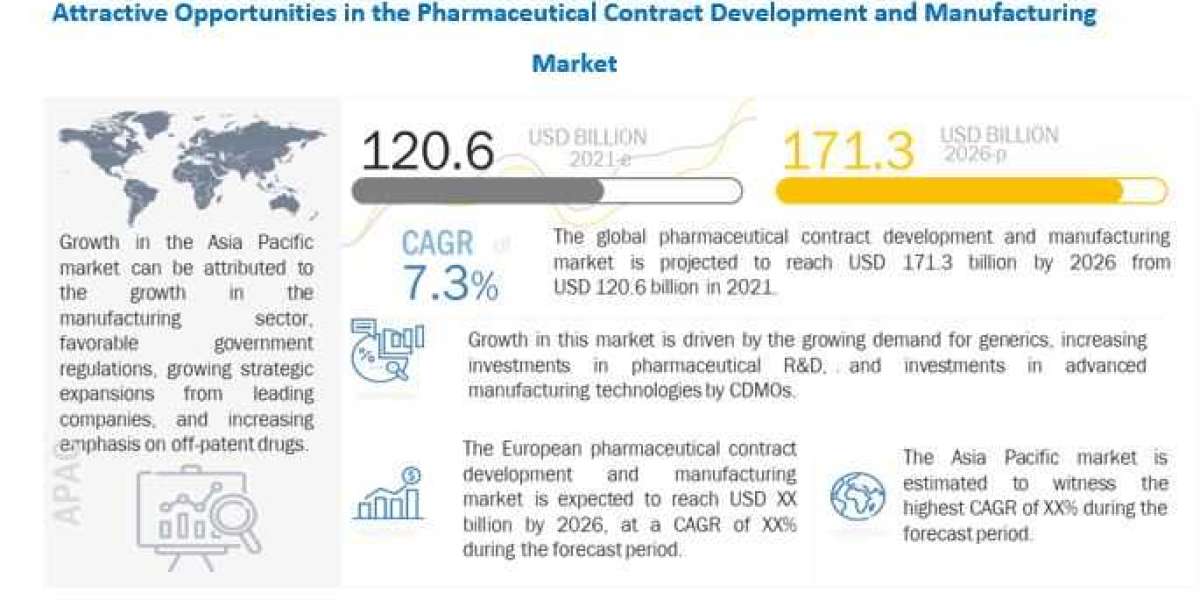
Currently, Pharmaceutical Contract Manufacturing Market is projected to reach USD 171.3 billion by 2026 from USD 120.6 billion in 2021, at a CAGR of 7.3% during the forecast period. Market growth is driven mainly by factors such as rising demand for generics, increasing investments in pharmaceutical RD, and investments in advanced manufacturing technologies by CDMOs. The increasing demand for biological therapies, growing focus on specialty medicines, growth in the nuclear medicines sector, and advancements in cell and gene therapies are also expected to offer market growth opportunities in the coming years.
Contract manufacturing is a process that involves outsourcing the production of a product to a third-party company. This allows pharmaceutical companies to focus on their core activities such as research and development and marketing, while outsourcing the production of the product to a CMO. This outsourcing model helps companies to reduce their overall production costs and improve their product quality.
The increasing demand for generic drugs, biologics, and orphan drugs is driving the growth of the pharmaceutical contract manufacturing market. The surge in demand for generic drugs has been largely attributed to their lower cost as compared to branded drugs, and the fact that they are often used for treating chronic diseases. Furthermore, the rising demand for biologics and orphan drugs, which are expensive and require specialized production processes, is also driving the growth of the market.
In addition to the rising demand for contract manufacturing services, the introduction of new regulations and guidelines is also expected to support the growth of the market. For instance, the US FDA has introduced the Quality System Regulation (QSR), which requires manufacturers to ensure that their products are safe, effective, and of good quality. This is likely to drive the demand for contract manufacturing services, as CMOs are better equipped to comply with stringent regulations.
Request for assumptions how numbers were triangulated.
https://www.marketsandmarkets.com/requestsampleNew.asp?id=201524381
The pharmaceutical manufacturing services segment accounted for the largest share of the service segment in the pharmaceutical contract development and manufacturing market in 2020.
On the basis of service, the pharmaceutical contract development and manufacturing market is broadly segmented into pharmaceutical manufacturing, biologics manufacturing, and drug development services. In 2020, the pharmaceutical manufacturing services segment accounted for the largest share of the pharmaceutical contract development and manufacturing market. The large share of this segment can be attributed to the limited finances of pharma manufacturers, capacity constraints, need for reductions in the time-to-market, complex manufacturing requirements, large investments required for establishing manufacturing facilities, and the growing pipeline, all of which have prompted the shift toward pharmaceutical contract manufacturing.
The rising demand for contract manufacturing is expected to drive the growth of the market in the years to come. The growing demand for generic, biologic, and orphan drugs, coupled with the introduction of new regulations and guidelines, is expected to drive the growth of the market. Furthermore, the cost savings associated with outsourcing production to CMOs is also expected to support the growth of the market.
Download an Illustrative Overview:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=201524381
Recent Developments
- In 2021, Samsung BioLogics (South Korea) entered into a Development and Manufacturing Agreement with Kineta (US) for Anti-VISTA Antibody Immunotherapy
- In 2021, Lonza Group (Switzerland) entered into an agreement with Aruvant Science (US) to carry out process development (one-time investigational gene therapy-ARU-1801) for sickle-cell treatment in the US
- In 2020, Catalent (US) acquired Skeletal Theray Support (Belgium). Under this acquisition, Catalent manufactured clinical material for Bone Therapeutics drug, ALLOB, an allogeneic osteoblastic cell therapy product
- In 2020, Recipharm AB (Sweden) acquired Consort Medical (UK) Bespak division dealing with inhalation and other devices
Content Source: