Key drivers of the stem cell therapy market include increase in stem cell research funding, expanding number of clinical trials related to stem cell therapies, and growing number of GMP-certified cell therapy production facilities. However, high costs associated with the development of stem cell therapy along with the ethical concerns related to embryonic stem cells are likely to hamper the market growth to a certain extent.
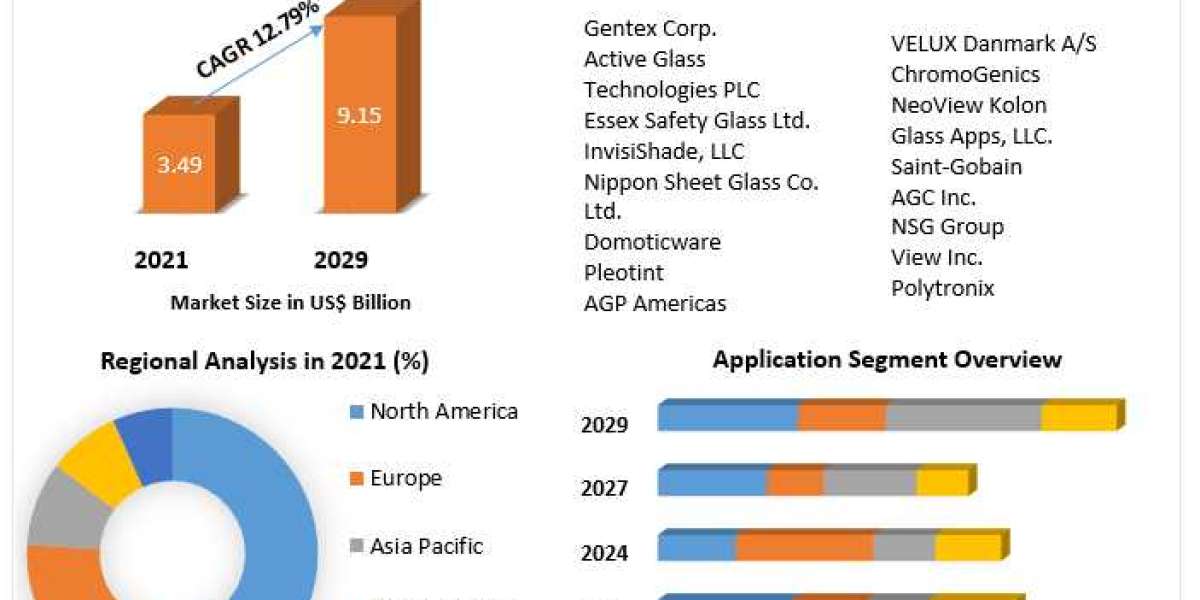
Stem Cell Therapy Market is projected to reach USD 558 million by 2027 from USD 257 million in 2022, at a CAGR of 16.8% during the forecast period, according to a new report by MarketsandMarkets™.
Browse in-depth TOC on "Stem Cell Therapy Market"
155 – Tables
43 – Figures
166 – Pages
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=48
KEY MARKET DYNAMICS
1 DRIVERS
1 Availability of funding for stem cell research
2 Increasing GMP certification approvals for cell therapy production facilities
3 Increasing clinical trials for stem cell-based therapies
2 RESTRAINTS
1 Ethical concerns related to embryonic stem cells
2 High cost of cell-based research
3 OPPORTUNITIES
1 Emergence of iPSCs as an alternative to ESCs
2 Growing demand for cell & gene therapies
4 CHALLENGES
1 Technical limitations associated with manufacturing processes
Market Segmentation
The global stem cell therapy market is segmented into adipose tissue-derived MSCs (mesenchymal stem cells), bone marrow-derived MSCs, placenta/umbilical cord-derived MSCs, and other cell sources.
Based on therapeutic application, the global stem cell therapy market is segmented into musculoskeletal disorders, wounds & injuries, cardiovascular diseases, surgeries, inflammatory & autoimmune diseases, neurological disorders, and other therapeutic applications.
Request For 10% Customization:
https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=48
Regional Analysis
The Asia Pacific region is estimated to grow at the highest CAGR in the stem cell therapy market during the forecast period. Japan and South Korea are the key revenue contributors of the Asia Pacific stem cell therapy market. Favorable government support for product approvals and the presence of major players in these countries are anticipated to drive the regional market growth.
Recent Developments:
- In July 2022, CORESTEM (South Korea) continued enrolling participants for the Phase 3 clinical trial of NeuroNata-R. This therapy has received conditional approval for treating ALS patients in South Korea
- In September 2020, Stemedica Cell Technologies received investigational new drug (IND) approval from the US FDA for intravenous allogeneic mesenchymal stem cells (MSCs) to treat moderate to severe COVID-19.
- In August 2020, Pluristem Israel) collaborated with Abu Dhabi Stem Cells Center (UAE) to develop stem cell therapies for COVID-19 treatment.
Top Key Players:
The stem cell therapy market is consolidated in nature with prominent players in the stem cell therapy market include Smith+Nephew (UK), MEDIPOST Co., Ltd. (South Korea), Anterogen Co., Ltd. (South Korea), CORESTEM (South Korea), Pharmicell Co., Ltd. (South Korea), NuVasive, Inc. (US), RTI Surgical (US), AlloSource (US), JCR Pharmaceuticals Co., Ltd. (Japan), Takeda Pharmaceutical Company Limited (Japan), Holostem Terapie Avanzate Srl (Italy), Orthofix (US), Regrow Biosciences Pvt Ltd. (India), and STEMPEUTICS RESEARCH PVT LTD. (India).