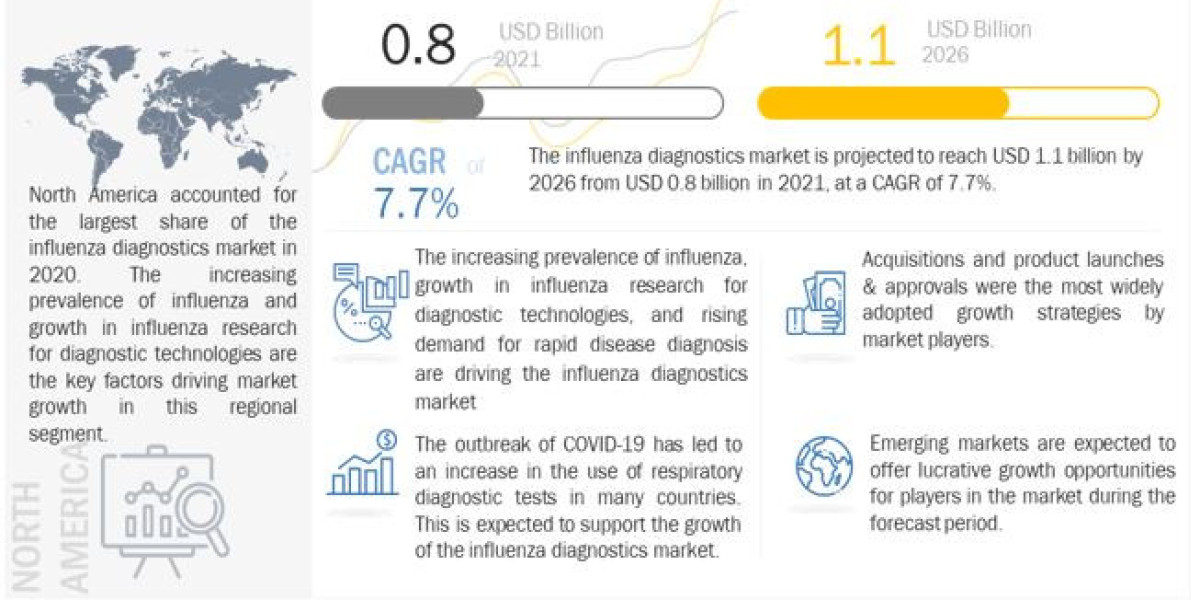
The report "Influenza Diagnostics Market is projected to reach USD 1.1 billion by 2026 from USD 0.8 billion in 2021, at a CAGR of 7.7% during the forecast period. Growth in the influenza diagnostics market is mainly driven by factors such as the increasing prevalence of influenza, Growth in influenza research for diagnostic technologies and Rising demand for rapid disease diagnosis. However, variabilities in test sensitivity and specificity and rising healthcare costs are the major factors hampering the growth of this market.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=222985562
Browse in-depth TOC on "Influenza Diagnostics Market”
154 – Tables
41 – Figures
193 – Pages
Key Market
The major players operating in this market are Danaher Corporation (US), Siemens Healthineers (Germany), Thermo Fisher Scientific, Inc. (US), F. Hoffmann-LA Roche AG (Switzerland), Abbott Laboratories, Inc. (Us), Hologic, Inc. (US), bioMérieux SA (France), Quidel Corporation (US), Becton, Dickinson and Company (US), Meridian Bioscience (US), GenMark Diagnostics, Inc. (US), Luminex Corporation (US), Tecanclinica Trading AG (Switzerland), DiaSorin SA (Italy), altona Diagnostics GmbH (Germany), SEKISUI Diagnostics (US), SA Scientific Ltd. (US), Coris BioConcept SPRL (Belgium), ELITech Group (France), Mast Group Ltd. (UK), Genome Diagnostics, Pvt. Ltd. (India), Germaine Laboratories, Inc. (US), Response Biomedical Corp. (Canada). Tauns Laboratories, Inc. (Japan) and 3B BlackBio Biotech India Ltd. (India).
The test kits and reagents segment accounted for the largest share of the influenza diagnostics market, by product segment, in 2020
Based on product and service, the influenza diagnostics market is segmented into test kits and reagents, instruments and other products. The test kits and reagents segment accounted for the largest share of the influenza diagnostics market in 2020. Factors such as growth in influenza research for diagnostic technologies and increasing prevalence of influenza are contributing for the growth of this market.
Molecular Diagnostic Tests segment to register the highest growth rate during the forecast period
The influenza diagnostics market is segmented into traditional diagnostic test (rapid influenza diagnostic tests, viral culture tests, direct fluorescent antibody test, serological tests) and molecular diagnostic tests (polymerase chain reaction, isothermal nucleic acid amplification tests(transcription-mediated amplification-based assay, loop-mediated isothermal amplification-based assay, nucleic acid sequence-based amplification tests, other isothermal nucleic acid amplification tests) and other molecular diagnostic tests. In 2020, the molecular diagnostic tests segment accounted for the highest growth rate. Factors such as the rising demand for rapid disease diagnosis drive this market and growth in influenza research for diagnostic technologies.
Request Sample Report: https://www.marketsandmarkets.com/requestsampleNew.asp?id=222985562
The Influenza Diagnostics Market encompasses the diagnostic tests used for the detection and diagnosis of influenza, commonly known as the flu. Influenza is a contagious respiratory illness caused by the influenza virus, and accurate and timely diagnosis is essential for effective disease management, patient care, and public health measures.
Key points regarding the Influenza Diagnostics Market, including regional dynamics, are as follows:
- Diagnostic Methods: Various methods are employed for detecting influenza viruses. Rapid influenza diagnostic tests (RIDTs), viral culture, polymerase chain reaction (PCR), immunofluorescence assays (IFA), and serological testing are among the commonly used diagnostic methods.
- Rapid Influenza Diagnostic Tests (RIDTs): RIDTs are widely used for quick influenza diagnosis. While these tests provide results within 15 minutes, their sensitivity and specificity are limited. However, newer RIDTs are being developed to enhance accuracy.
- Molecular Diagnostic Tests: Polymerase chain reaction (PCR) is a highly sensitive and specific method for detecting influenza viruses. PCR tests identify viral genetic material in patient samples, providing accurate results. Real-time PCR assays are often utilized for rapid diagnosis.
- Point-of-Care Testing: Point-of-care tests (POCT) are gaining importance in influenza diagnostics. These tests can be performed near the patient, allowing for rapid diagnosis and immediate treatment decisions.
- Market Growth: The global influenza diagnostics market is experiencing steady growth due to factors such as the increasing incidence of influenza infections, the need for rapid and accurate diagnostics, and growing awareness of early disease detection. The market includes products from various companies and diagnostic laboratories.
- Regional Dynamics: a. North America: The North American market holds a significant share in the influenza diagnostics market. Factors such as the high incidence of influenza, advanced healthcare infrastructure, favorable reimbursement policies, and robust research and development activities contribute to the market growth in this region.
- Europe: Europe also represents a substantial market for influenza diagnostics. The region's well-established healthcare systems, government initiatives for disease surveillance, and a large patient pool drive market growth. Additionally, the presence of key market players contributes to the development of innovative diagnostic solutions.
- Asia Pacific: The Asia Pacific region exhibits significant potential for growth in the influenza diagnostics market. The rising population, increasing awareness about the importance of early diagnosis, improving healthcare infrastructure, and expanding access to healthcare services contribute to market expansion. Moreover, the region's large and densely populated countries present a substantial market opportunity.
- Latin America, Middle East & Africa: These regions are also witnessing growth in the influenza diagnostics market. Improving healthcare infrastructure, increasing investments in healthcare, and rising awareness about infectious diseases contribute to market development. However, certain challenges, such as limited access to healthcare services and resource constraints, may impact market growth to some extent.
Prominent companies in the influenza diagnostics market include Danaher Corporation (US), Siemens Healthineers (Germany), Thermo Fisher Scientific, Inc. (US), F. Hoffmann-LA Roche AG (Switzerland), Abbott Laboratories, Inc. (Us), Hologic, Inc. (US), bioMérieux SA (France), Quidel Corporation (US), Becton, Dickinson and Company (US), Meridian Bioscience (US), GenMark Diagnostics, Inc. (US), Luminex Corporation (US), Tecan Trading AG (Switzerland), DiaSorin SA (Italy), altona Diagnostics GmbH (Germany), SEKISUI Diagnostics (US), SA Scientific Ltd. (US), Coris BioConcept SPRL (Belgium), ELITech Group (France), Mast Group Ltd. (UK), Genome Diagnostics, Pvt. Ltd. (India), Germaine Laboratories, Inc. (US), Response Biomedical Corp. (Canada). Tauns Laboratories, Inc. (Japan) and 3B BlackBio Biotech India Ltd. (India)
These companies offer a range of diagnostic tests and systems for influenza detection.
It's important to note that the influenza diagnostics market is dynamic, with ongoing advancements in technology. For the most up-to-date information, it is advisable to refer to market research reports, industry publications, and consult healthcare professionals and experts.
Top of Form
North America is the largest regional market for influenza diagnostics market
The global influenza diagnostics market is segmented into five major regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2020, North America accounted for the largest share of the influenza diagnostics market. The large share of this region can be attributed to growth in influenza research for diagnostic technologies and rising demand for rapid disease diagnosis.
Report Link: Influenza Diagnostics Market
Recent Developments:
- In May 2021 Becton, Dickinson and Company (US) received FDA approval for the BD Veritor Plus System, which is used to detect SARS-CoV-2, influenza A, and influenza B.
- In March 2021, Abbott Laboratories, Inc. (US) received emergency use authorization (EUA) from the FDA for a laboratory PCR assay that detects and differentiates SARS-COV-2, flu A, flu B, and RSV in one test.
- In February 2021, Becton, Dickinson and Company (US) received approval from the FDA for the emergency use authorization (EUA) for a new molecular diagnostic test for both SARS-CoV-2 and influenza A+B.
Report Link: Influenza Diagnostics Market
About MarketsandMarkets™
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies’ revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their pain points around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 Micro Quadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets’s flagship competitive intelligence and market research platform, "Knowledge Store" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
Email: [email protected]
Research Insight: https://www.marketsandmarkets.com/ResearchInsight/influenza-diagnostic-market.asp
Visit Our Website: https://www.marketsandmarkets.com/
Content Source: https://www.marketsandmarkets.com/PressReleases/influenza-diagnostic.asp
Related Reports:

![Harmony Glow CBD Gummies [Latest Offers 2024-25 Updated]](https://thewion.com/upload/photos/2024/11/7ZIdKhDMd7gkys1pUM94_08_17a47125cd02f3818c93bbf5e36ae0d1_image.jpg)
